Explain How Sublimation and Deposition Are Different
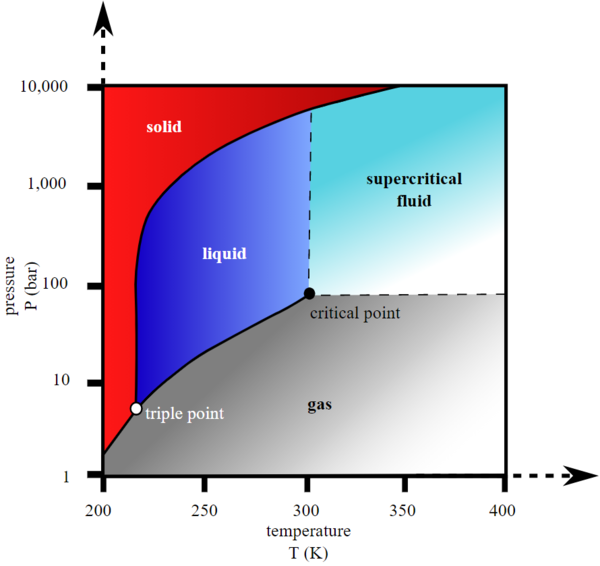
Each line separating the different phases is where they are in equilibrium. These temperature-pressure data pairs correspond to the sublimation or deposition points for water.

Art The Phase Changes Of Matter Include Melting Freezing Evaporation Condensation Deposition And Sublima Matter Science States Of Matter Changes In Matter
Explain the construction and use of a typical phase diagram.
. All else being kept identical until deposition of the back contact either 1 poor back-contact passivation or 2 a modification of the absorber or. Thus if we place a frozen sample in a vacuum with a pressure less than 020 kPa ice will sublime. 12062021 Create an account.
For example on the line separating liquid water and solid water or. Water in an open container at room temperature slowly disappears evaporation. The thickness of sediments and the age of fossils in the seafloor d.
Which of the following changes of state is an exothermic process. The reverse process of sublimation is deposition. Relative atomic mass The mass of an atom relative to that of carbon-12.
Changes in the Earths magnetic field. In this process the matter is present in the gaseous state and as the reaction occurs it gets converted into solid state without being converted into a liquid. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
Describe the supercritical fluid phase of matter. How this data supports the idea of seafloor spreading. Use phase diagrams to identify stable phases at given temperatures and pressures and to describe phase transitions resulting from changes in these properties.
Patterns of polarity in the seafloor. If we could zoom in on the solid-gas line in Figure 2 we would see that ice has a vapor pressure of about 020 kPa at 10 C. Learn about the state changes of matter including freezing melting boiling condensation sublimation and deposition.
No correct response. A sub-zero temperature is needed to achieve this state. In your answer be sure to address the topics belowUse complete sentences.
Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. Explain how the differing ages of rocks on the oceans floor supports the theory of plate tectonics. In higher altitudes and south and north poles the vapours of.
This is approximately the sum of the number of. In the previous module the variation of a liquids equilibrium vapor pressure with temperature was described.

Art The Phase Changes Of Matter Include Melting Freezing Evaporation Condensation Deposition And Sublima Matter Science States Of Matter Changes In Matter

Sublimation Vs Deposition Youtube

Sublimation And Deposition Energy Education

Difference Between Sublimation And Deposition Compare The Difference Between Similar Terms
No comments for "Explain How Sublimation and Deposition Are Different"
Post a Comment